Rabies Vaccine Serial Numbers
Manufacturer: Intervet/Merck Animal Health RABIES VACCINE Killed Virus Annual Revaccination: Dogs, Cats & Ferrets IN THE ABSENCE OF A VETERINARIAN-CLIENT-PATIENT RELATIONSHIP, FEDERAL REGULATIONS PROHIBIT THE RELABELING, REPACKAGING, RESALE, OR REDISTRIBUTION OF THE INDIVIDUAL CONTENTS OF THIS PACKAGE. DIRECTIONS FOR USE - PLEASE READ CAREFULLY FOR ANIMAL USE ONLY Product Description Nobivac ® 1-Rabies vaccine is for vaccination of healthy dogs, cats and ferrets as an aid in preventing rabies. The vaccine is prepared from cell-culture-grown, chemically-inactivated rabies virus. The seed virus is a highly immunogenic, fixed strain of rabies virus which originated from Louis Pasteur’s original isolate in 1882.
Zone E Complete A Side Singles Rarities. Appendix 4: Commonly asked questions about vaccination. PDF printable version of Appendix 4: Commonly asked questions about vaccination of the 1. RABIES VACCINATION CERTIFICATE NASPHV FORM 51 (revised 2007) RABIES TAG #. Vaccine Serial (lot) Number NEXT VACCINATION 1 Yr USDA Licensed Vaccine.
The inactivated virus is formulated with a highly purified adjuvant and is packaged in liquid form. Disease Description Rabies is a worldwide, high mortality disease affecting mammalian species. Wild animals are common vectors of the disease and the major source of transmission to humans and domestic animals. Despite successful attempts over the years to reduce the incidence of rabies, recent published reports indicate that in the U.S. More than 30,000 people undergo treatment every year for possible exposure. 1 Domestic animals are the major source of exposure for humans. Since 1980, the most commonly reported rabid domestic animals have been cats, cattle and dogs.
In 1990, a total of 4,881 cases of animal rabies were reported to the Center for Disease Control by all 50 states, the District of Columbia and Puerto Rico. 2 Susceptibility to rabies varies according to pet species.
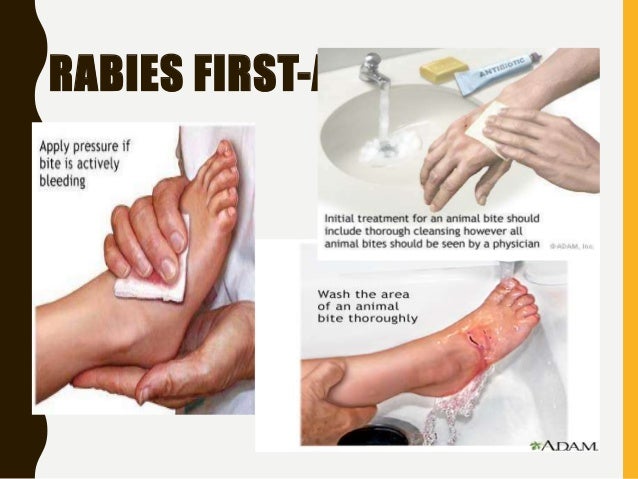
Rabies is not a treatable disease and suspect pets are usually quarantined until a clinical diagnosis is made, at which time they are destroyed. The route of infection can be oral, respiratory, or parenteral. Following infection, a paralytic syndrome ensues, emerging as either the “furious” or “dumb” form. “Furious rabies” is characterized by unusual aggression; “dumb rabies” by lethargy and a desire to avoid contact.
Respiratory failure is the immediate cause of death. Safety And Efficacy Because Nobivac 1-Rabies vaccine is produced on an established cell line, it has safety advantages over inactivated brain-origin rabies vaccines. Tissue-origin vaccines contain extraneous protein in addition to rabies antigen that can lead to autoimmune disease. The established cell line used in Nobivac 1-Rabies has been extensively tested for freedom from contaminating agents. In addition, use of an established cell line yields a vaccine of consistent potency from serial to serial. Nobivac 1-Rabies has proven to be uniformly safe in experimental tests and no significant adverse reactions were reported in extensive clinical trials of the vaccine. Duration of immunity studies, conducted in accordance with federal regulation and under U. Open Cdr Files In Gimp. S.
Department of Agriculture direction, demonstrated that a 1 mL dose met federal guidelines for protection of dogs, cats and ferrets against virulent challenge administered more than a year after vaccination. PRECAUTIONS 1. Store at 2°-8°C (35°-46°F). Prolonged exposure to higher temperatures may adversely affect potency. Do not freeze. Use entire contents when first opened. Do not mix with other products.
In case of human exposure, contact a physician. This product has not been tested in pregnant animals. Sterilized syringes and needles should be used to administer this vaccine. Contains gentamicin and thimerosal as preservatives. As with many vaccines, anaphylaxis may occur after use. Initial antidote of epinephrine is recommended and should be followed with appropriate supportive therapy.
This product has been tested under laboratory conditions and shown to meet all Federal Standards for safety and ability to immunize normal healthy animals. This level of performance may be affected by conditions of use such as stress, weather, nutrition, disease, parasitism, other treatments, individual idiosyncrasies or impaired immunological competency. These factors should be considered by the user when evaluating product performance or freedom from reactions. Directions Following the advice of the American Veterinary Medical Association on Vaccination Principles, an appropriate revaccination program for individual animals should be made based upon veterinarian-client-patient relationships. General Directions: Shake well.
Aseptically administer 1 mL subcutaneously. Dogs may be vaccinated intramuscularly or subcutaneously. Primary Vaccination: Healthy dogs, cats and ferrets should receive a single dose at 3 months of age or older. A repeat dose should be administered 1 year later. Revaccination: Annual revaccination with a single dose is recommended.
REFERENCES 1. Kaplan M.M., Koprowski H.: Rabies, Austr Vet Pract 10:208-215, 1980.
Uhaa I.J., Mandell E.J., Whiteway R., et al: Rabies surveillance in the United States during 1990. Vet Med 200:920-929, 1992. FOR ANIMAL USE ONLY Manufactured by: Zoetis Inc., Kalamazoo, MI 49007, USA VLN 190/PCN 1905.24 Distributed by: Intervet Inc., Omaha, NE 68103 USA VLN 165A 1 800 224-5318 (USA) 40018578 Code 5x10 mL Vials of Vaccine 0857 CPN: 1047166.5 MERCK ANIMAL HEALTH Intervet Inc. 2 GIRALDA FARMS, MADISON, NJ, 07940 Customer Service: 800-521-5767 Order Desk: 800-648-2118 Technical Service (Companion Animal): 800-224-5318 Technical Service (Livestock): 800-211-3573 Fax: 973-937-5557 Website: www.merck-animal-health-usa.com Every effort has been made to ensure the accuracy of the Nobivac 1-Rabies information published above. However, it remains the responsibility of the readers to familiarize themselves with the product information contained on the US product label or package insert.